Consider the reaction between butyllithium and ethanol, a captivating topic that unveils the intricacies of organic chemistry. This reaction, involving a strong base and a nucleophile, holds immense significance in various synthetic applications. Delving into its mechanism, products, influencing factors, and practical uses, this discourse aims to provide a comprehensive understanding of this fundamental reaction.
Introduction
Butyllithium (BuLi) is a strong, non-nucleophilic base commonly used in organic synthesis. Ethanol (EtOH) is a polar, protic solvent. The reaction between butyllithium and ethanol is a classic example of a nucleophilic substitution reaction.
The purpose of analyzing this reaction is to understand the mechanism, products, and factors affecting the rate and selectivity of the reaction. This knowledge is important for designing and optimizing synthetic procedures involving butyllithium and ethanol.
Mechanism of the Reaction: Consider The Reaction Between Butyllithium And Ethanol
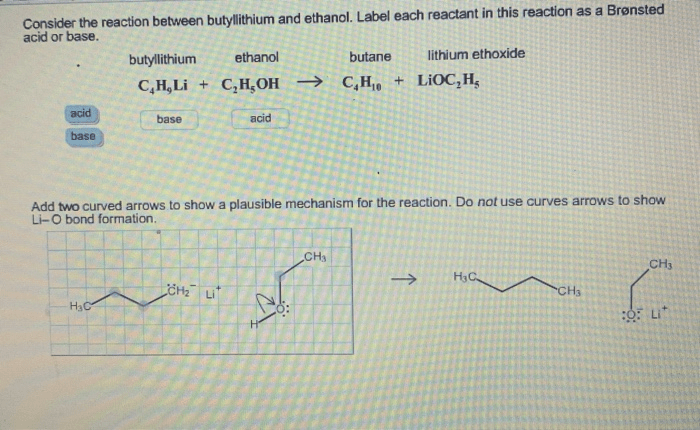
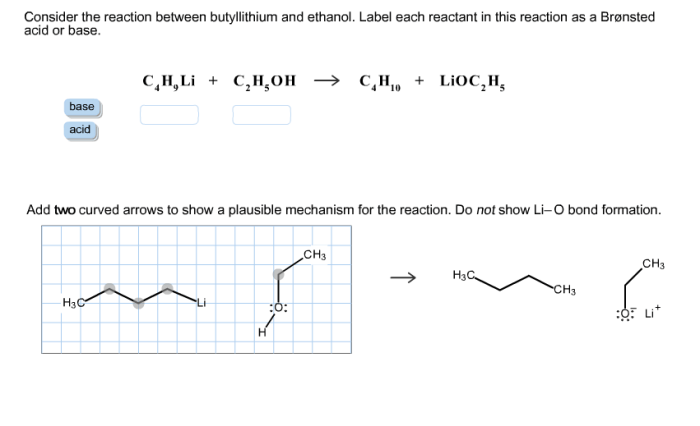
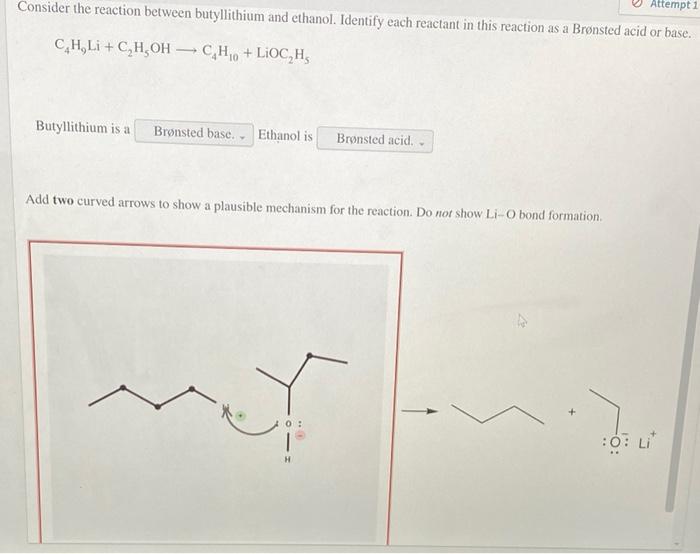
The reaction between butyllithium and ethanol proceeds via a nucleophilic substitution mechanism. The butyllithium acts as a base, abstracting a proton from the ethanol molecule. This generates an ethoxide ion (EtO-), which then attacks the butyllithium molecule, displacing the lithium ion (Li+).
The overall reaction can be represented as follows:
BuLi + EtOH → EtO- + BuH + Li+
Products of the Reaction
The major product of the reaction between butyllithium and ethanol is butyl ethyl ether (BuOEt). This is formed by the nucleophilic attack of the ethoxide ion on the butyllithium molecule.
A minor product of the reaction is lithium ethoxide (LiOEt). This is formed by the reaction of the ethoxide ion with the lithium ion.
Factors Affecting the Reaction
The rate and selectivity of the reaction between butyllithium and ethanol are affected by several factors, including:
- Temperature:The reaction rate increases with increasing temperature.
- Solvent:The reaction rate is faster in polar, aprotic solvents such as tetrahydrofuran (THF) than in nonpolar solvents such as hexanes.
- Concentration:The reaction rate increases with increasing concentrations of butyllithium and ethanol.
Applications of the Reaction
The reaction between butyllithium and ethanol is used in a variety of organic synthesis applications, including:
- Alkylation:Butyllithium can be used to alkylate a variety of compounds, including aldehydes, ketones, and esters.
- Dehydrohalogenation:Butyllithium can be used to dehydrohalogenate alkyl halides, forming alkenes.
- Metalation:Butyllithium can be used to metalate a variety of compounds, including arenes and heteroarenes.
Safety Considerations
Butyllithium and ethanol are both hazardous chemicals. Butyllithium is a pyrophoric solid that reacts violently with water. Ethanol is a flammable liquid. The following safety precautions should be taken when working with these chemicals:
- Butyllithium should be handled in a dry, inert atmosphere.
- Ethanol should be stored in a cool, well-ventilated area.
- Both chemicals should be disposed of properly according to local regulations.
Question & Answer Hub
What is the role of butyllithium in this reaction?
Butyllithium serves as a strong base, facilitating the deprotonation of ethanol.
What are the major products of this reaction?
The major products are the alkoxide salt and ethane.
How does temperature affect this reaction?
Elevated temperatures favor the formation of the alkoxide salt, while lower temperatures promote the formation of ethane.